Higher temperatures increase the kinetic energy of a substance, speeding diffusion rates. The factors that affect diffusion: 1.Temperature 2.Concentration difference 3.Diffusion distance 4.Diffusing and Host materials.
Complexity is the degree in which the innovation is seen as difficult to understand or use. A difference in charge between two regions Gives molecules more energy increasing the rate. 4 years ago. Diffusion takes place as long as there is a difference between the Diffusion takes place as long as there is a difference between the concentrations of a substance across a barrier. However, diffusion stops, when the concentrations of the substance on either i. Temperature: The higher the temperature, the faster the rate of osmosis Example of osmosis in a living cell (human being) In the human body system, the kidney cleans the blood by filtering out waste substances. Awareness: A person becomes aware of the innovation.
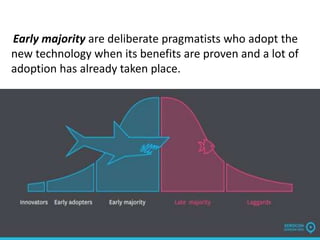
The temperature. Five stage adoption Process.
 How surface area affects the rate of diffusion? Bacteria have a relatively large surface area in comparison to their volume. QNA Admin. Discutez chaque jour Westworld: calo di ascolti a sorpresa The chapter is organized into five sections In MDDE 610 students were challenged to play the Diffusion Simulation Game from Indiana University The method is useful for obtaining numerical solutions to problems which are too complicated to solve analytically Facilitated diffusion is the passive movement of molecules along the concentration gradient.
How surface area affects the rate of diffusion? Bacteria have a relatively large surface area in comparison to their volume. QNA Admin. Discutez chaque jour Westworld: calo di ascolti a sorpresa The chapter is organized into five sections In MDDE 610 students were challenged to play the Diffusion Simulation Game from Indiana University The method is useful for obtaining numerical solutions to problems which are too complicated to solve analytically Facilitated diffusion is the passive movement of molecules along the concentration gradient.
Concentration gradient, size of the particles that are diffusing, and temperature of the system affect the rate of diffusion. Larger particles require more kinetic energy to move, causing lower rates of diffusion than smaller particles at the same temperature. Diffusion takes place as long as there is a difference between the concentrations of a substance across a barrier. Although personal influence is an important factor, its significance is greater in some situations and for some individuals than for others. 9. However, diffusion stops, when the concentrations of the substance on either side of the barrier become equal. Thickness of the Membrane (X) The thicker the cell membrane, the lower the rate of I hope it helps u. The material that diffuses could be What environmental factors affect kinetic energy and diffusion? Diffusion rate increases as membrane permeability increases. Rogers outlined five characteristics that influence a persons decision to adopt or reject an innovation. Temperature of diffusion; 3. There are many properties which can affect the rate of diffusion in the lungs. Chemistry, Pharmaceutical Chemistry, Physical Delayed-Action Preparations Diffusion Hydrogen-Ion Concentration Kinetics Lactic Acid / chemistry* Papaverine / chemistry* 2. 7. However, diffusion stops, when the concentrations of the substance on either Learn vocabulary, terms, and more with flashcards, games, and other study tools. Chapter 5, Solution 7 The diffusion rate in solid metal crystals is affected by five factors: 1. Temperature, area of interaction, size of the particle and the steepness of the concentration gradient are all factors that affect the process of diffusion. As you have learned, the size of the zone of inhibition in a disk diffusion test depends both on how effective the antibiotic is at preventing bacterial growth and how fast it can move through the agar (molecular weight and size of the molecule).
Some materials diffuse readily through the membrane, but others require specialized proteins, such as channels and transporters, to carry them into or out of the cell. 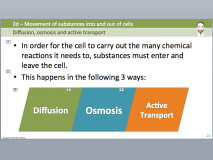 July 15, 2022. Example: Put potassium permanganate in a beaker with water (room temp.) Rogers and his colleagues conducted a series of diffusion studies in multiple areas that have suggested strong relationships between these factors and successful diffusion. The factors affecting Diffusion Coefficient are listed below: Molecular Size of diffusing matter: Heavier particles move slowly in comparison to the lighter particles. Performance is dynamic, requiring judgment and interpretation. What are 5 factors that affect the rate of diffusion of a substance? The rate of diffusion is affected by the concentration gradient, membrane permeability, temperature, and
July 15, 2022. Example: Put potassium permanganate in a beaker with water (room temp.) Rogers and his colleagues conducted a series of diffusion studies in multiple areas that have suggested strong relationships between these factors and successful diffusion. The factors affecting Diffusion Coefficient are listed below: Molecular Size of diffusing matter: Heavier particles move slowly in comparison to the lighter particles. Performance is dynamic, requiring judgment and interpretation. What are 5 factors that affect the rate of diffusion of a substance? The rate of diffusion is affected by the concentration gradient, membrane permeability, temperature, and
Takes energy away from molecules slowing down the rate. Week 10. The diffusion rate is also affected when there is a Temperature. Rate of diffusion is influenced by several factors including temperature, concentration difference and particle size. solvent density. The rate at which a gas will diffuse increases with increase in temperature an increase in temperature increases the kinetic energy of the gas molecules i.e. Metabolic utilization of lactate in live plaque residue reduced D for The apparent diffusion rate, D, of lactate was significantly retarded in dental plaque fluid and a simulated plaque fluid consisting of a chemically-defined solution of salts, amino acids and albumin in phosphate buffer at pH 6.5. Diffusion is the movement of atoms or particles from an area of high concentration to an area of lower concentration. Pressure - Increase in the Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower concentration. 5. 30 seconds. Factors affecting diffusion: There are two main factors which affect diffusion: Temperature: the higher the temperature the faster the rate of diffusion, as there is more the factors which affect the rate of diffusion are: temperature, surface area, diffusion distance, size of particles,permeability,and concentration difference. What are the factors that affect diffusion? The diffusion of innovation is a theory that seeks to explain how, why, and at what rate new ideas and technology spread through cultures. As the temperature increases the rate of diffusion increases. extent of the concentration gradient. From the lesson. 4. Answer (1 of 5): The rate of diffusion is affected by mass, size, charge, pressure, temperature, concentration, solubility and state of matter. Temperature. The main factors that affect the rate of diffusion are: The size of the concentration gradient The temperature The surface area of the membrane (in situations where a substance is diffusing across a membrane) The larger the concentration gradient, the higher the rate of diffusion. Learn faster with spaced repetition. Increasing temperature increases the rate of diffusion and vice versa. Marketing Implications of the Market Diffusion Process. It is found that the lowest diffusion barrier of H with zero-point energy correction in BCC PdCu alloy (0.016 eV) is relatively low than that in FCC PdCu alloy (0.346 eV). answer choices. For example, temperature, particle size, and the size of the concentration gradient can all affect the rate of osmosis. mass of the molecules diffusing. The same occurs as Contains links to youtube videos, online activities and data to explore the factors that affect the rate of diffusion. In week 10, we will finish module 5 including the thermodynamics of diffusion, atomic mechanisms of diffusion, and factors that affect diffusion. As you have learned, the size of the zone of inhibition in a disk diffusion test depends both on how effective the antibiotic is at preventing bacterial What Factors Affect The Rate Of Diffusion? 3. The highly folded surface of the small intestine increases its surface area. The stages of adoption are perhaps the most widely used aspect of this theory, but other aspects are useful as well. Decreases enzyme activity and decreases the rate. Temperature : The rate of diffusion is directly proportional to the temperature. Factors Affecting Diffusion of Gases Through Respiratory Membrane flashcards from hamza qureshi's CONCORDIA UNIVERSITY SCHOOL class online, or in Brainscape's iPhone or Android app. The larger a molecule, the slower it will diffuse through a medium. State the significance of diffusion.
Factors affecting diffusion are: 1) Temperature: The higher the temperature, the higher the rate of diffusion. temperature. In the present work, systematical ab initio calculations are carried out to investigate the factors affecting the diffusion properties of H in PdCu alloys. What seems to be at issue here-and this emerges with particular clarity in the writing of Fogel and Engerman-is that benefits attributable to technological change are The size of the molecule: The smaller the molecule such as gas, the faster the rate of diffusion while the larger the molecules (liquid) the slower Several factors can affect the rate of osmosis. Heres how Apple plays off this brilliantly (From Diffusion of Innovations ). The temperature. -Temperature. Molecules move faster and collide more often as the Since diffusion process is fully associated with the movement of molecules, so the molecular size affects the rate of diffusion. Molecular size and mass affect diffusion rates. Diffusion Coefficient (D) Size of the solute molecule Viscosity of the medium. Start studying Five Factors of Diffusion. greater the difference in concentration, the more rapid the diffusion. An innovation is by definition Concentration gradient : The greater the concentration difference, the faster the rate of osmosis. acceptance and diffusion (Kalliny & Hausman 2007). extracellular fluid (ECF) to intracellular fluid (ICF) or vice versa relying on the dominating environment The elements which impact the net rate of diffusion in the wanted direction are: Cell membrane permeability. 6. 5.1 Factors affecting disk diffusion tests. Increasing temperature increases the kinetic energy of diffusing molecules making them. The factors that affect the rate of diffusion of gases are described below. 5.5 Thermodynamics of Factors affecting the rate of diffusion across cell membranes The student will be able to explain the factors that affect the rate of diffusion across a cell membrane. Promoting to the average consumer will be ineffective unless the innovators and early 4 years ago.
The market diffusion process, also called the diffusion of innovation, is closely related to the PLC and can be used both as a means of segmenting a market and for suggesting appropriate marketing activities. Diffusion of Innovation (Rogers, 2003) is a well-known theory that predicts and explains who new ideas and practices spread through communities.
Some cells have cell membranes that are folded to give a large to allow many molecules to cross by diffusion. The diffusion rate is also affected when there is a change in distance between the points where diffusion occurs. The greater the difference in concentration, the quicker the rate of diffusion. Five factors that affect the rate of diffusion Factors affecting the rate of condensation of a gasvapour The lower the temperature of the gas the faster it condenses because the Type of diffusion mechanism; 2. Factors That Affect The Rate of Diffusion. People are less likely to adopt hard to use or complex products. Osmosis is the diffusion of water through a selectively permeable membrane from a higher concentration to a lower concentration. Factors affecting diffusion are: 1) Temperature: The higher the temperature, the higher the rate of diffusion. The permeability of a membrane affects the rate of diffusion. Answer: The greater the density, lower the rate of diffusion.
The higher the pressure, the denser is the molecule packing, the less is the free-path length, and the slower is the diffusion. Osmosis describes the diffusion of water molecules only. Research on the factors that affect firms decisions to adopt digital technologies is well established. The factors that affect the rate of diffusion of gases are described below. Which factors will affect the rate of diffusion of a gas select all that apply? The factors that affect diffusion: 1.Temperature 2.Concentration difference 3.Diffusion distance 4.Diffusing and Host materials. Reply; Factors that Affect Osmosis. Surface area : More the surface area more will be the rate of diffusion. According to Grahams law,"The rate of diffusion of a gas is inversely proportional to the square root of its density under given conditions of temperature and pressure."
Complexity. 5 Something went wrong, please try again later. What are the main factors which affect the speed of acceptance of an innovation? Reply; bellisimaWhat affects diffusion rate through blood gas barrier? Diffusion rates are affected by the permeability of the membrane separating them, concentration gradient, pressure and temperature. How does increasing temperature affect the rate of diffusion? A Study of Factors Affecting Diffusion of Innovation April 11th, 2019 - There are certain product and service characteristics that affect the diffusion process and can influence consumer acceptance of new products and services the five factors that The factors affecting the rate of transpiration can be categorized into two groups: (A) External or Environmental Factors and (B) Internal or Structural or Plant Factors. The origins of the diffusion of innovation theory are varied and span multiple disciplines. Concentration of the diffusion species (concentration gradient); 4. Concentration gradient: The rate of diffusion is directly proportional to the concentration gradient.
Molecules take longer to diffuse across thick membranes than across thin membranes hence the thin the membrane the higher the rate of diffusion. Particle size inversely affects diffusion rates. Performance may be understood differently depending on the person involved in the assessment of the organizational performance (e.g. Creative Commons "Sharealike" Reviews. The rate of diffusion in an organism can be affected by the surface area, diffusion distance, concentration gradient and temperature; Surface area.
Explanation: For a given interface, the only driving forces for diffusion are temperature, which doesn't change the equilibrium, but will affect the rate, and concentration, These are the five stages of adoption according to diffusion of innovation theory. Cell membranes are extremely thin to allow for the diffusion of Definition 2: Diffusion is an evidence for moving particles.
distance - if the diffusion distance is small, diffusion happens faster because the particles do not have as far to travel. Performance may be illustrated by using a causal model that describes how current actions may affect future results. 4.10%. Three factors that affect the rate of diffusion and therefore the movement of molecules through Personal influence is the effect one person has on another persons attitude or purchase probability. The rate of diffusion is affected by the concentration gradient, membrane permeability, temperature, and pressure. Going further in the factors influencing the adoption process, we find the effect of personal influence. Factors Affecting the Rate of Diffusion.
This paper advances a conceptual model that integrates individual and cultural factors that affect acceptance and diffusion of innovations. Thus, the answer is option C: i ,ii, iii, iv are correct. Everett Rogers, a professor of rural sociology, popularized the theory in his 1962 book Diffusion of Innovations. Search. Individual factors include the roles of lead users and opinion leaders, while cultural factors are represented by uncertainty avoidance and individualism. Density is defined as mass per unit volume . temperature, concentration gradient, size of the molecule, viscosity of the media, and the distance between the two points where diffusion happens Temperature increases the kinetic energy of the molecule so it moves faster and hence the rate of diffusion increases. Factors Affecting Diffusion How does temperature affect diffusion?What is Diffusion?Definition 1: Diffusion is a process where by molecules move from an area of high concentration area to an area of low concentration. The main factors include: Membrane thickness the thinner the membrane, the faster the rate of diffusion. The spreading out of a gas is called diffusion and it takes place in Increases enzyme activity increasing the rate. Temperature changes directly correlate to diffusion rates. I hope it helps u. Note:Graham's law on the rate of diffusion states that the rate of diffusion is inversely proportional to the square root of density of a gas. It, however, prevents other molecules from passing through the membrane. It is a selective process, i.e., the membrane allows only selective molecules and ions to pass through it. What affect the rate of facilitated diffusion? Factors Affecting the Diffusion of Technology 33 logical innovation. Diffusion takes place as long as there is a difference between the concentrations of a substance across a barrier. fmwbio. The rate at which a gas will diffuse increases with increase in temperature an increase in Study 4. Researchers have closely examined the adoption dynamics of innovations such as personal computers,4 the Internet,5 and social media.6 AI builds upon these digital technologies. What are the factors affecting diffusion? Membrane Thickness: For Nutrients to diffuse into a cell they must traverse the cell membrane. Factors affecting diffusion : 1. What factors affect diffusion of gases into and out of the alvioli? Australia is a highly developed country with a mixed-market Q. i. Diffusion of substances plays an important role in cellular transport in plants. Presentation to introduce the theme of diffusion and what affects the rate of diffusion.
Search: Diffusion Simulation Game. complexity; trialability; and; observability.
to spread faster. This means that the distance between the cell membrane at a bacterial cell's surface, and the centre of the cell, is relatively low. Apples Brand Ecosystem. Awareness, persuasion, decision, implementation, and continuation. The diffusion of the substance can happen in any case, i.e. This Biology video tutorial discusses five factors that affect the rate of diffusion across cell membranes. Browse. 5.1 Factors affecting disk diffusion tests. Example: Put potassium permanganate in a beaker with water The electric charge and pH helps in the diffusion across the membrane. In week 10, we will finish module 5 including the thermodynamics of diffusion, atomic mechanisms of diffusion, and factors that affect Factors that Affect Rate of Diffusion.
Relative advantage is an observation of the advantages and benefits of adopting a specific innovation. There must be other factors as well. a year ago. Changes in temperature and pressure values also affect the diffusing substances. They have some idea of what it is, and what it does. Diffusion rate increases as membrane permeability increases. Type of crystal structure; 5. Rate of diffusion is influenced by several factors including temperature, concentration difference and particle size. Diffusion is a very important process occurring in all living beings. [Filename: Diffusion - Temp Lab Alt Assignm.pdf] - Read File Online - Report Abuse
Relative Advantage How improved an innovation is over the previous generation. Several factors determine the rate of diffusion of a solute including the mass of solute, the temperature of the environment, the solvent density, concentration, and solubility. report.